Chemical cleaning and scale inhibition are essential for the longevity of water systems. The presence of scale can lead to a loss of heat transfer efficiency, system clogging, and increased energy costs. Chelants are commonly included in water treatments to prevent scale build-up and keep system efficiency high and costs low.
What are chelants?
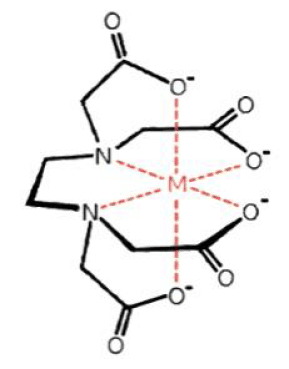
Chelants are organic compounds that form water soluble complexes with metal ions. These complexes have high stability and “trap” the metal ions, preventing them from reacting with other molecules and forming insoluble salts and precipitates. As a result, the chelants aid in the prevention of scale and corrosion.
The word chelation comes from the Latin word of chele, which means “claw”. You can imagine an image of the claws of a crab wrapping around the metal ion to form a ring-like structure. In this case, the two claws of the crab are forming a bidentate complex. The same concept applies with chelant chemistry.
What are common chelants used in water treatment?
The most common chelant used in water treatment is EDTA (ethylenediaminetetraacetic acid). The less common chelants are based on gluconates. In some instances, citric acid is used as a chelating agent, as well. Although NTA (Nitrilotriacetic Acid) has its advantages as a chelant, such as not attacking the boiler metal, it is a known carcinogen and is mostly avoided.
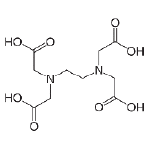
EDTA will chelate the metal ions of iron, copper, aluminum, calcium and magnesium. EDTA reacts with the free ions in the system initially but can react with the system metal if overfed for a period of time. The order in which EDTA will chelate metal ions can be predicted by looking at their stability constants, with a higher constant meaning preferential binding to EDTA first. So, if a system contains Fe3+, Cu2+, Fe2+ and Ca2+, any EDTA added will complex the Fe3+ first. The Ca2+ will be the last to be complexed.
Table 1. Stability constants of common metal ions with EDTA
| Metal Ion | Stability constant w/ EDTA (log K) |
|---|---|
| Fe3+ (ferric) | 25.10 |
| Cu2+ (Copper) | 18.78 |
| Al3+ (Aluminum) | 16.40 |
| Fe2+ (ferrous) | 14.30 |
| Ca2+ (Calcium) | 10.65 |
| Mg2+ (Magnesium) | 8.79 |
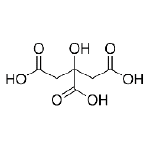
Citric Acid is a naturally occurring chelating agent that solubilizes metals such as iron and copper. It is used in low pH, high hardness water to help chelate Ca2+ and Mg2+ and it is often found in industrial cleaners.
Gluconates have been widely used in the cleaner industry for removal of corrosion byproducts and to prevent corrosion. In water treatment applications, they have an advantage over EDTA and NTA due to their lack of toxicity, high stability and high solubility. Gluconate is an exceptional chelating agent for divalent and trivalent ions, and, when it reacts with sodium hydroxide, it reverts to sodium gluconate, which has nine (9) times the chelating capacity of EDTA. Gluconates also react with metal surfaces to form a protective layer by adsorption to the metal surface.
Table 2. Chelating capacity of chelants with ferric iron
| Chelant | Chelating Capacity |
|---|---|
| Sodium Glucoheptonate | |
| Sodium Gluconate | |
| EDTA |
Where do we use Chelants?
Historically, chelants have commonly been found in boiler applications, but they can also be found in other systems related to cooling, agriculture, and other cleanings.
Boiler feed water, in many cases, has some cationic salts present from calcium, magnesium and iron. These salts will concentrate in the presence of other boiler water components during the process of steam generation. Since these materials are inversely soluble with increasing temperature, the normally water-soluble calcium, magnesium and iron lose the ability to remain in solution and precipitate as a hard solid. This inverse solubility allows these salts to form an insulating deposit on the boiler metal surfaces. EDTA can be used in boiler applications to create water-soluble salts with these cations. This soluble salt formation reduces the level of suspended solids in the boiler.
EDTA and glucoheptonate are also found in the ethanol industry to maximize production of the final product. Although metals may be beneficial to microorganisms during the process of glucose fermentation, excess metals will decrease production of ethanol. As such, the chelants are able to tie up metals and allow yeast to have maximum efficiency to produce ethanol. After the fermentation process, distillation converts 15% ethanol to 95% ethanol. Many times, the distillation equipment is subject to scale formation from impurities so chelants are used to maintain system efficiency, as well. Finally, chelants are found in industrial cleaners to be used on much of the equipment, from fermenters to heat exchangers, cleaning up the scale and biofilms.
How Do We Use Chelants in Boiler Systems?
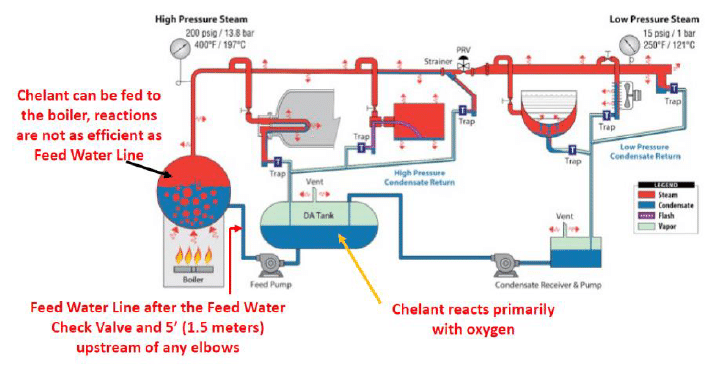
Internal treatment of boiler systems is the most common application for chelants. In boilers, a chelant should be fed through a stainless-steel quill placed in the feed water line. The injection point must be downstream of the feed water pump and at least five feet up stream from any bend in the feed water line. Proper installation will prevent copper loss from the feed water pump impeller as well as any erosion of the feed water piping at elbow bends. This allows maximum contact between the chelant and the feed water impurities to be treated.