Author
Mike Bertrang – VP of Sales
PROS of Industrial Glycol Loops
Freeze Protection – One of the main benefits of glycol loops is that they depress the freeze point when mixed with water. This helps prevent pipes and equipment from freezing in cold environments.
Effective Heat Transfer – Glycol mixtures can provide good thermal conductivity, allowing for efficient heat transfer between the coolant and the environment. This makes them suitable for cooling systems, industrial processes, and HVAC systems.
Non-corrosive – When properly maintained, glycol mixtures (especially when inhibited) are less likely to corrode metal parts in the system compared to water-only systems. This is important for the longevity of equipment and infrastructure.
Large Systems Scalability – Glycol loops are flexible and can be used in both small and large systems, from refrigeration units to large-scale industrial cooling setups.
Safe Contact with Equipment – In most cases, glycol is safer for direct contact with machinery compared to other chemicals like ammonia, which is commonly used in industrial refrigeration.
Versatility – Glycol mixtures are widely used in various industrial sectors such as food and beverage, pharmaceuticals, and chemical processing due to their adaptability to different temperature and process conditions.
Enhanced System Efficiency – Glycol-based loops can help maintain consistent temperatures over time, improving the overall efficiency of the cooling system.
Biostatic – When the glycol concentration in water exceeds around 25% by volume, both ethylene glycol and propylene glycol are biostatic and the need for biocide is eliminated.
CONS of Industrial Glycol Loops
Viscosity – Glycol mixtures tend to have higher viscosity than water. This can lead to higher energy consumption for pumping, especially in large systems, as more effort is needed to move the thicker fluid through the loop.
Cost – Glycol and its additives can be more expensive than water, both in terms of initial setup costs and ongoing maintenance (e.g., regular fluid replacement, inhibitor additives).
Environmental Impact – If not handled properly, glycol can be harmful to the environment, particularly ethylene glycol which is toxic to aquatic life. Propylene glycol is less toxic but still requires proper disposal and management.
Maintenance Requirements – Glycol systems require periodic maintenance to check the concentration levels, pH, and inhibitor concentrations. Over time, glycol mixtures can degrade, and the system must be replenished or replaced.
Potential for Overheating – Glycol is less effective at heat transfer than water at higher temperatures. At higher temperatures, the heat transfer efficiency of glycol loops may decrease, requiring larger or more frequent cooling efforts.
Fouling and Scaling – Glycol systems can experience fouling (buildup of debris or contaminants) and scaling (mineral deposits) if not properly maintained, which can reduce heat transfer efficiency and lead to system damage.
System Complexity – Because glycol loops involve a mixture of fluids, they can make the system more complex, with additional components like expansion tanks, temperature sensors, and pressure monitoring equipment, which can increase the potential for system failures if not properly managed.
Degradation – Over time, glycol can degrade due to heat, exposure to air, or contamination. This degradation reduces the ability of the glycol to prevent freezing and can contribute to corrosion and other issues in the system.
Considerations
Glycol loops are a good option for industrial cooling and temperature regulation, offering freeze protection and efficient heat transfer. However, they come with trade-offs like higher maintenance, costs, and environmental considerations. The choice to use a glycol loop depends on the specific requirements of the application, the temperature conditions, and the ability to maintain and manage the system effectively.
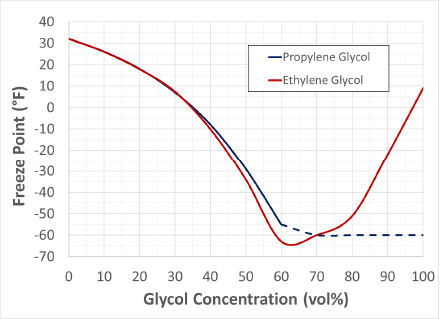
Below is graph showing freeze point depression of water mixed with ethylene glycol or propylene glycol. Above 60 vol%, propylene glycol will maintain a freeze point around -60°F while the ethylene glycol curve continues to increase. Due to these diminishing returns, it is not typical to run high concentrations of glycol in closed loops.