Matt LaBrosse, PhD
One key drawback to azoles is their susceptibility to degradation from oxidizers, particularly an oxidizing biocide like bleach. Best practices require interlocking feed pumps, for example, to ensure an azole inhibitor and bleach are not fed simultaneously. Halogen-resistant azole (HRA) compounds have been promoted in the past, but data and case studies supporting their performance can be limited. As part of continuous product improvement, the Apex Tech Team is proactively looking to investigate any new raw materials that may have an advantage in finished products (i.e., improved performance, lower usage cost, new/innovative chemistry). For example, a new compound was identified back in 2022 and investigated in the legacy Apollo lab with claims of resistance to halogen degradation. After an extensive experimental review, this compound was branded YellowGuard and implemented in several products. It will also be included in many products within the new Apex product line.
Comparing yellow metal corrosion inhibitors experimentally in the lab with LPR methods

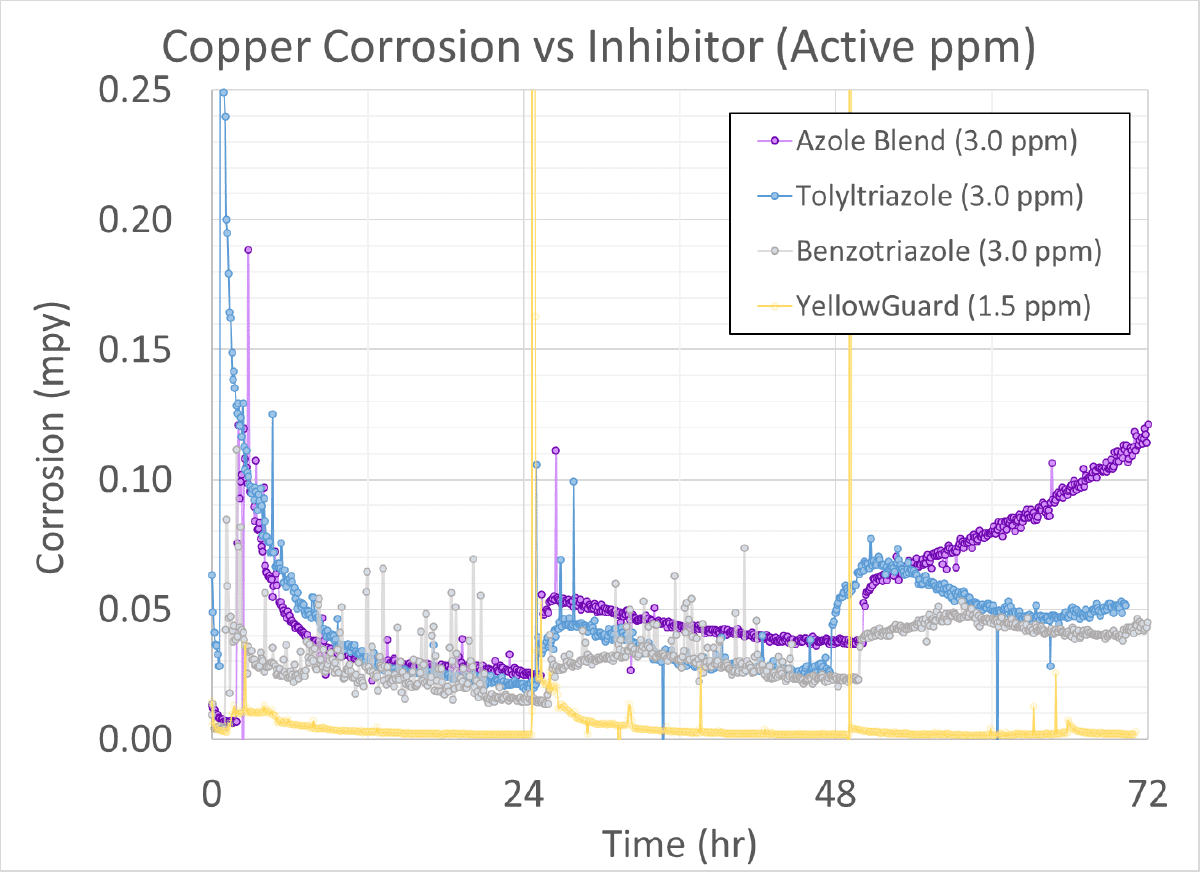
The image to the right shows an LPR setup for running a copper coupon, the small copper cylinder in the center of the glass jar. This is a 1-liter apparatus filled with a synthetic brine meant to simulate a typical Midwest hard water. Each run is aerated and therefore open. However, water loss is negligible in each run. Using this setup, YellowGuard was compared against 3 commonly used azoles found in many finished products: SAM azole blend (a.k.a. Copper Bullet), tolyltriazole, and benzotriazole.
For this experiment, each inhibitor was dosed into the synthetic brine at the start of the run and allowed to equilibrate for 2 hours. At that point, the system was dosed with 12.5% bleach up to 1.0 ppm free chlorine. The system was then allowed to recover and equilibrate. The system was similarly dosed again with 12.5% bleach at the 24-hour and 48-hour mark, for a total of 3 oxidizing biocide additions. No additional inhibitor treatment was added during the run.
How does Apex’s YellowGuard inhibitor perform against common azoles?
Looking at the graph on the next page, YellowGuard performed very well against the azoles. Even with a lower dosage of 1.5 ppm active (as compared to 3.0 ppm active from the other azoles), it consistently had a corrosion rate near zero. YellowGuard also proved to have a more tenacious protective layer with the copper coupon, which can be seen by the YG recovering quickly to near-zero corrosion after each bleach addition. The other 3 azoles show increased corrosion rates after each bleach addition, which is evidence of degradation from the oxidizing biocide.
It should be noted these results are relative, comparing the YG to the other azoles. The corrosion rates for the other azoles are all < 0.10 mpy which is considered Excellent by AWT best practices.
What are the pros and cons of using YellowGuard?
YellowGuard is currently being used in many accounts throughout the legacy Apollo territory. Feedback from the field has been positive, particularly in accounts trying to lower their copper corrosion numbers while needing to maintain higher free and total chlorine residuals on their oxidizing biocide program. YellowGuard is also comparable in cost to the more commonly used azoles. YellowGuard requires a high pH during blending, so it is currently limited to high pH products only. As such, products that are reformulated with YellowGuard may also require a higher pH that will reclassify the product as DOT Hazardous (i.e., corrosive).
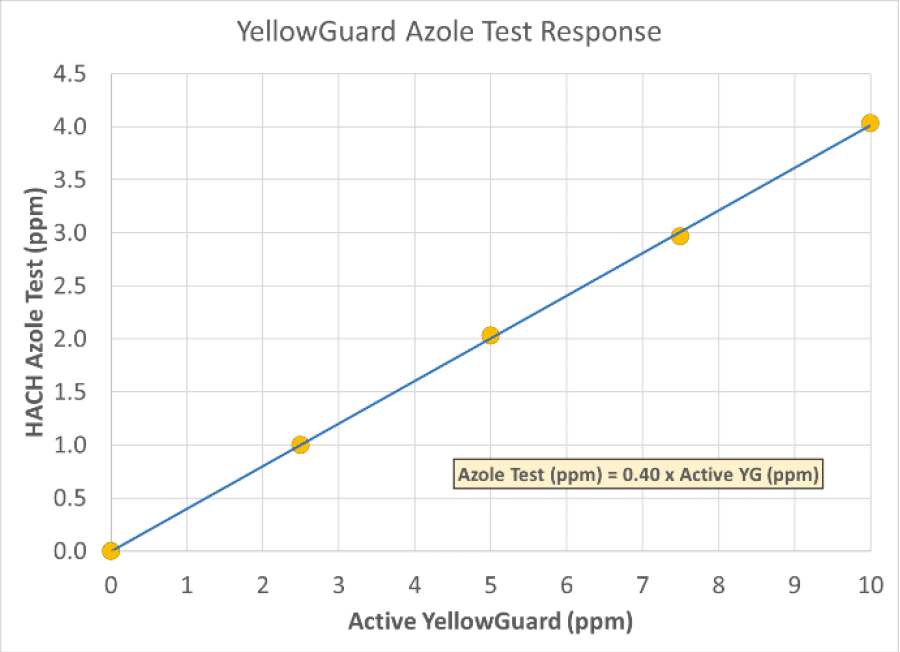
How is YellowGuard tested in a treated system?
While the chemistry is different than traditional azoles like benzotriazole and tolyltriazole, YellowGuard will still respond to an azole test at a lower intensity. Below is correlation curve that was developed in the lab for YellowGuard using HACH Method 8076 on the DR900 handheld. The graph shows a correction factor of 0.40 is needed on the test result.
For example, for a YellowGuard active concentration of 1.50 ppm in the treated system water sample, the DR900 test will output 0.60 ppm as azole.